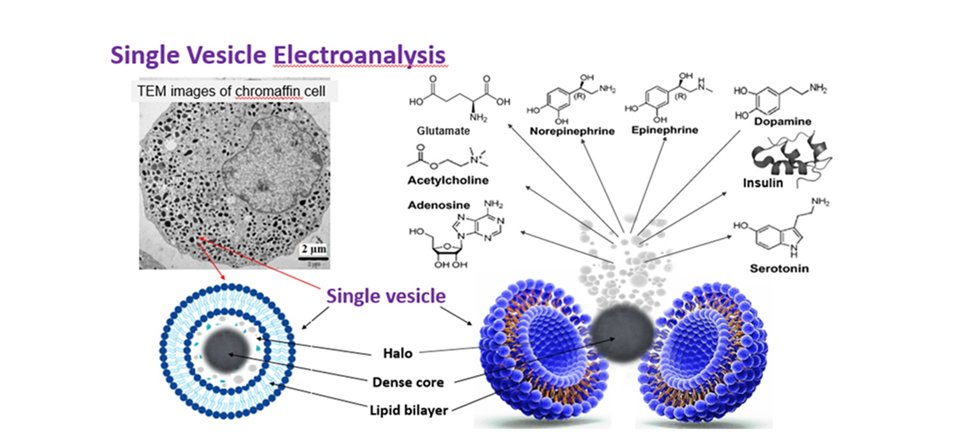
Research
Methods to Study Single Cells, Cell Membranes, Single Vesicles, and Exocytosis
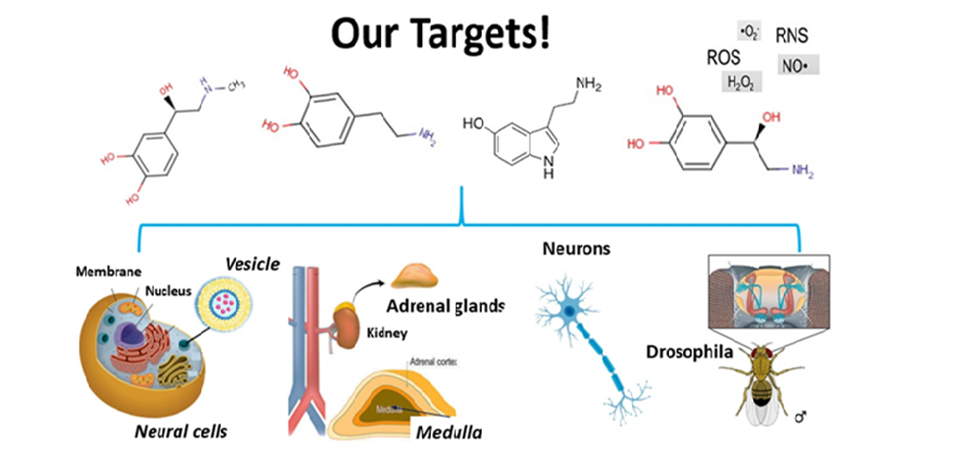
Focusing on the neuronal process of exocytosis, the Ewing group has pioneered small-volume chemical measurements at single cells, electrochemical detection for capillary electrophoresis, novel approaches for electrochemical imaging of single cells, and new electrochemical strategies to separate individual nanometer vesicles from cells and quantify their contents. The group also pioneered many methods for the development and application of mass spectrometry imaging for subcellular and neurochemical analysis.
In the last 10 years, we have worked along several themes of cell and organelle research. New methods to measure the contents of nanometer vesicles in vitro and in vivo have been pioneered (JACS and Angew Chemie 2015, respectively) and used to propose a mechanism for the cognitive changes in the “chemobrain” observed in cancer patients (Angew Chemie 2016). We then used this new technology to show that zinc, anaesthetics, methylphenidate, cocaine, and modafinil change vesicle content and the fraction released in exocytosis providing a potential mechanism to explain how drugs affect learning impairment and improvement (Angew Chemie 2017, 2019, 2020) and demonstrated that exocytosis in live neurons in Drosophila release a very small fraction of their content (Angew Chemie 2020). They developed a new paradigm for plasticity and partial release with electrochemical measurements and repetitive stimulations (PNAS 2019) and compared this to cell membrane structure, with a follow up to discover an effect of iron deficiency on plasticity (Angew Chemie 2022). They have shown that partial release is clearly evident in several cell systems including in gut epithelium cells, pancreatic beta cells (Angew Chemie 2x2021). They also used these methods to discover and identify reactive oxygen species in stress granules (Angew Chemie 2021). They have developed analytical methods to simultaneously measure chemical messengers inside and outside a single cell (Chemical Science 2021) and simultaneously count molecules in the halo and dense-core of nanovesicles by regulating dynamics of vesicle opening (Angew Chemie 2022). NanoSIMS has been used to directly visualise partial release with regular exocytosis (ACS Nano 2022), in subvesicular nanocompartments (Int J Mol Sci 2023), and to correlated vesicle size and partial release (Angew Chemie 2023). Preliminary data with organelles includes electrochemical methods used to find transmitter molecules in exosomes (JACS 2023) and stress granules effect on vesicles to cause homotypic fusion thereby modulating release (Angew Chemie 2024).
In addition to the work described above, the group has built a unique mass spectrometry imaging laboratory, second to none in SIMS imaging with an IonTof V instrument, Ionoptika J105 3D Chemical Imager, an AB Sciex Qstar equipped with a C60 ion gun, a Bruker Ultraflextreme MALDI instrument, and now the Cameca NanoSIMS (added recently).
Some Current Research Directions
● Vesicle impact electrochemical cytometry (VIEC). We are working to examine the mechanism of vesicle opening in VIEC and determine the role of lipids in this mechanism, which could help us understand the effect of lipids on pore opening in exocytosis.
● New methods for vesicle impact electrochemical cytometry (VIEC). Here we propose new nano fabricated approaches for VIEC, one for high throughput vesicle analysis, and the other to quantify substance in the protein dense core of a single vesicle.
● Intracellular vesicle impact electrochemical cytometry. We plan to develop new approaches with intracellular vesicle impact electrochemical cytometry (IVIEC) combined with amperometric measurements of exocytosis to measure the fraction of vesicular messenger released and, importantly, we will use these platforms to examine and quantify how lipids (and zinc, which is correlated with learning in mammals) affect the fraction of messenger released during exocytosis, an important step in development of methods to explore the effect of lipids on plasticity.
● Chemically dissecting nanometer vesicles..We are working with nanoelectrochemistry and chaotropic ions to chemically dissect and spatially locate nanometer organelles across cells for chemical analysis.
● Vesicle structure/function: NanoSIMS combined with IVIEC. We propose to apply our new combined technology with 1) the NanoSIMS, probing to 40 nm spatial resolution and imaging the substructure of vesicles with 2) dynamic amperometric detection of exocytotic release and IVIEC of vesicle content in cells.
● To spatially measure single entities in situ across cells.We are developing nanoprobes that can be tracked in the cell for local subcellular measurements.
● New intracellular measurements.We are wroking to establish protocols to measure transmitter exchange (communication) between organelles in the cell.
● Organelle-organelle interactions. We are working to build a unified theory of how organelles involved in cellular communication interact through experiments in organoids and Drosophila melanogaster.
● Model of effectors for short-term memory. Our plan is to assemble the measurements above into a model that will show the simplest chemical steps in the initiation of synaptic plasticity and from there the strengthening to a short-term enhancement otherwise considered short-term memory.
REFERENCES
1. Ostrowski, S. G., et al. Mass Spectrometric Imaging of Highly Curved Membranes During Tetrahymena Mating.Science, 305 (2004) 71-73.
2. Kurczy, et al. Mass Spectrometry Imaging of Mating Tetrahymena: Changes in Cell Morphology Regulate Lipid Domain Formation.Proc. Natl Acad Sci USA, 107 (2010) 2751-2756.
3. Dunevall, J. et al. Characterizing the catecholamine content of single mammalian vesicles by collision-adsorption events at an electrode.J Am Chem Soc, 137 (2015) 4344-4346.
4. Phan, N. T. N., Fletcher, J. S., Ewing, A. G. Lipid Structural Effects of Oral Administration of Methylphenidate in Drosophila Brain by Secondary Ion Mass Spectrometry Imaging J Am Chem Soc, 137Anal Chem, 87 (2015) 4063-4071.
5. Li, X., et al., A. G. Quantitative Measurement of Transmitters in Individual Vesicles in the Cytoplasm of Single Cells with Nanotip Electrodes.Angew Chem Int Ed, 54 (2015) 11978-11982.
6. Majdi, S. et al. Electrochemical Measurements of Optogenetically Stimulated Quantal Amine Release from Single Nerve Cell Varicosities in Drosophila Larvae. Angew Chem Int Ed, 54 (2015) 13609-13612.
7. Najafinobar, N. et al. Excited Fluorophores Enhance the Opening of Vesicles at Electrode Surfaces in Vesicle Electrochemical Cytometry. Angew Chem Int Ed, 55 (2016) 15081-15085.
8. Li, X., Dunevall, J. & Ewing, A. G. Using Single-Cell Amperometry to Reveal How Cisplatin Treatment Modulates the Release of Catecholamine Transmitters during Exocytosis. Angew Chem Int Ed, 55 (2016) 9041-9044.
9. Lovric, J. et al. Nano Secondary Ion Mass Spectrometry Imaging of Dopamine Distribution Across Nanometer Vesicles.ACS Nano, 11 , (2017) 3446-3455. doi:10.1021/acsnano.6b07233.
10. Ren, L. et al. Zinc regulates chemical transmitter storage in nanometer vesicles and exocytosis dynamics measured by amperometry. Angew Chem Int Ed, 56 (2017) 4970-4975
11. Ren, L. et al. The Evidence for Open and Closed Exocytosis as the Primary Release Mechanism.Q Rev Biophys, 49 (2016) doi:10.1017/S0033583516000081.
12. Li, X., Dunevall, J., Ewing, A. G. Quantitative Chemical Measurements of Vesicular Transmitters with Electrochemical Cytometry.Acc Chem Res, 49 (2016) 2347-2354.
13. J. Dunevall, A. Larsson, S. Majdi, A. G. Ewing, Vesicle impact electrochemical cytometry compared to amperometric exocytosis measurements,Current Opinion in Electrochemistry, (2017) DOI: org/10.1016/j.coelec.2017.07.005.
14. X. Li, L. Ren, J. Dunevall, D. Ye, H.S. White, M.A. Edwards, A.G. Ewing, On the Nanopore Opening at Flat and Nano-Tip Conical Electrodes during Vesicle Impact Electrochemical Cytometry,J ACS Nano , in press (DOI: 10.1021/acsnano.8b00781).
15. N.T.N. Phan, X. Li and A.G. Ewing, Measuring synaptic vesicles using cellular electrochemistry and nanoscale molecular imaging,Nature Reviews Chemistry,, 1 (2017) DOI:10.1038/s41570-017-0048.